In the modern era, we often need to pay more attention to the consistency and excellence of the products and services we use daily. This was not the case at the beginning of the 20th century when quality control in manufacturing was far from reliable.
Today, thanks to the pioneering work in business problem-solving and analytical frameworks, businesses can achieve and maintain high-quality standards.
This blog explores how these Quality Control practices have evolved and impacted today’s industries.
What is Quality Control (QC)?
Quality Control (QC) is vital in ensuring that products and services meet a set quality standard. It involves testing and measuring these products or services to confirm they align with the desired level of excellence.
The concept of ‘quality’ might vary, but in QC, it’s all about meeting established standards and providing value. This process enables businesses to assess, maintain, and enhance the quality of their offerings, ensuring customer satisfaction and trust.
The primary goal of Quality Control is twofold. Firstly, it aims to ensure products are as consistent as possible. Uniformity is key to maintaining a reliable brand image and customer experience. Secondly, QC strives to minimize errors and inconsistencies.
This is achieved by monitoring and inspecting products or services at different stages of production or delivery. QC isn’t just about fixing problems; it’s also focused on preventing defects by implementing control measures and improving processes, thus ensuring a higher standard of product or service delivery.
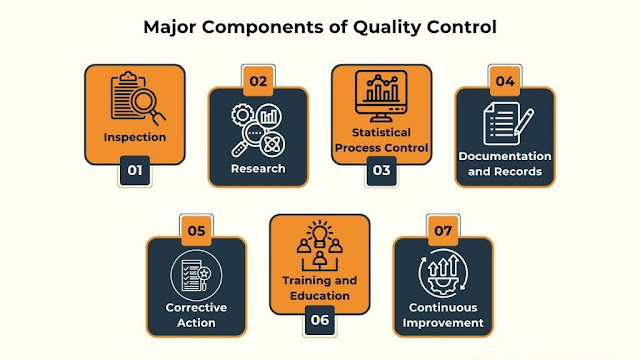
Major Components of Quality Control
Quality Control (QC) is integral to maintaining high standards in product and service delivery. It encompasses a variety of components, each playing a crucial role in ensuring that the final output meets or exceeds the expected quality.
The components include:
- Inspection: This involves regularly examining products, materials, or services to identify any defects, non-compliance, or deviations from the established quality standards
- Testing: Various tests assess performance, functionality, or other characteristics, ensuring products or services meet quality expectations
- Statistical Process Control (SPC): This employs statistical methods to monitor and control production processes, keeping them within acceptable quality limits
- Documentation and Records: Keeping detailed and accurate records of inspections, tests, and corrective actions is essential for maintaining traceability and accountability
- Corrective Action: Whenever quality issues are identified, appropriate measures are taken to rectify them and prevent their recurrence
- Training and Education: Empowering employees with the necessary skills and knowledge is vital for effectively maintaining quality standards
- Continuous Improvement involves analyzing data and feedback to refine and enhance the quality management system
Quality Control is closely intertwined with Quality Assurance (QA). While QC is focused on identifying and correcting defects, QA is about preventing these defects by establishing robust processes and procedures. Together, they form the backbone of an organization’s approach to quality management, crucial for meeting customer expectations and regulatory standards.
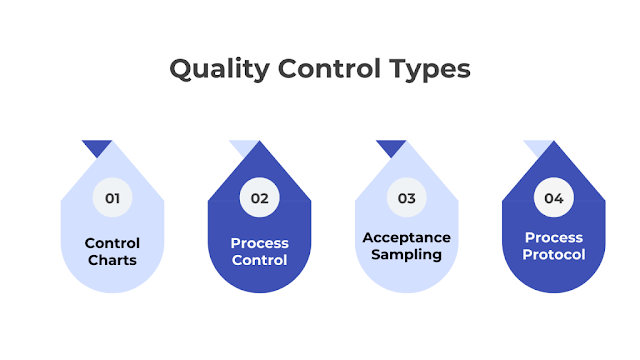
Quality Control Types
Quality Control (QC) is a diverse and dynamic field, with its methods varying significantly across different industries. The type of QC employed often depends on the specific requirements and risks associated with a particular sector.
For instance, industries like food and pharmaceuticals, where consumer safety is paramount, might lean towards more scientific and rigorous QC methods.
In contrast, fields like education or coaching might adopt a more qualitative approach, focusing on holistic improvement. Regardless of the industry, QC is fundamentally about meticulous attention to detail and robust research methodology.
Several key types of Quality Control methods are commonly used across various industries:
Control Charts
Control charts are graphical representations that track process changes over time. By statistical analysis, businesses can determine whether their manufacturing or service processes are within the control limits.
Process Control
This involves continuous monitoring and adjusting processes to ensure consistent quality and improved performance. It often includes technical methods like feedback loops and industrial controls, especially in manufacturing.
Acceptance Sampling
This method uses statistical sampling to decide whether a batch of products meets the overall manufacturing standards, helping make informed decisions about product quality.
Process Protocol
This approach maps out the design and implementation processes, setting evaluative indicators for each step to ensure that each production or service delivery phase meets quality standards.
Beyond these, the approach to QC can also vary in terms of internal versus external monitoring.
Some companies establish internal QC divisions to oversee their products and services continuously. In contrast, others may rely on external bodies for quality assessment, especially in industries like food and pharmaceuticals with stringent regulations and safety.
Importance and Benefits of Quality Control
Quality Control (QC) is more than just a set of procedures; it’s a pivotal aspect of business operations that brings numerous benefits to the company and its customers. By enforcing QC measures, organizations can enhance their product and service quality and gain a competitive edge in the market.
Here are some key benefits of implementing Quality Control:
- Customer Satisfaction: QC ensures products and services consistently meet or exceed customer expectations, fostering higher satisfaction and loyalty. This leads to repeat business and positive word-of-mouth referrals.
- Defect Prevention: Early identification and correction of issues in the QC process prevent defects, reducing costly recalls or rework. This also enhances the overall reliability of the product or service.
- Cost Reduction: Implementing QC measures leads to reduced waste, lower production costs, and improved efficiency, contributing to significant cost savings. This financial efficiency can be reinvested into further improvements or innovation.
- Compliance and Regulations: Adherence to industry standards and regulatory requirements through QC helps avoid legal issues and penalties, ensuring smooth business operations and maintaining corporate integrity.
- Brand Reputation: High-quality outputs build a positive brand image, enhancing reputation and market competitiveness. A strong reputation is invaluable for long-term business growth and success.
- Increased Efficiency: QC optimizes processes, leading to higher productivity and streamlined operations, faster delivery times, and increased capacity.
- Risk Mitigation: Through rigorous testing and inspections, QC helps identify potential risks and hazards, allowing businesses to address them proactively and maintain a safe environment for employees and customers.
- Continuous Improvement: A focus on QC encourages ongoing enhancement of products, services, and processes, fostering a culture of innovation and adaptability within the organization
- International Competitiveness: High-quality products enabled by QC can facilitate entry into global markets, increasing a company’s competitiveness on an international scale and opening up new revenue streams
- Customer Retention and Loyalty: Satisfied customers, as a result of high-quality products or services, are more likely to stay loyal and recommend the brand, contributing to the long-term success and sustainability of the business
Quality Control is crucial for maintaining high standards, minimizing risks, and ensuring a sustainable competitive advantage in today’s rapidly evolving business landscape. It is the foundation upon which superior products and services are delivered, ensuring customer satisfaction and fostering brand loyalty.
Quality Control Vs. Quality Assurance
While often used interchangeably, Quality Control (QC) and Quality Assurance (QA) are distinct concepts in quality management.
Quality Control primarily focuses on the end product or service, ensuring it meets specific quality criteria and complies with necessary specifications. This involves direct actions like inspection, testing, and correcting defects in products or services. QC is about identifying and addressing problems after they occur.
On the other hand, Quality Assurance encompasses all the processes and actions necessary to provide confidence that quality requirements will be fulfilled. It’s more about managing and improving processes to prevent defects in the first place. QA is a proactive approach, emphasizing establishing a comprehensive quality management system and continuous improvement.
The relationship between the two is that QC is a part of the broader QA process. QA sets the standards and procedures to ensure quality, while QC involves the operational techniques to fulfill these standards.
As professionals grow in their careers, they may shift focus from QC to QA, understanding that ensuring quality is not just about fixing problems but also about creating systems that prevent them.
QA programs and departments are crucial for management, customers, and inspectors to guarantee that products meet all quality requirements and safety regulations.
Example of Quality Control (QC)
To illustrate Quality Control (QC) in action, consider a stuffed toy manufacturer focusing on a teddy bear product. The company has identified eight key parameters to control quality, aiming for consistency in look and feel across all teddy bears.
The QC team, which may vary in size depending on the scale of operations, is responsible for ensuring each teddy bear meets these standards.
Their tasks would typically include:
- Material Quality: Checking the fabric and stuffing material for consistency and quality.
- Size and Shape: Ensuring each teddy bear matches predefined size and shape specifications.
- Color: Verifying the color of the fabric and any additional elements like ribbons or buttons.
- Stitching Quality: Inspecting the stitching for durability and uniformity.
- Safety Standards: Ensure the toy meets safety regulations, especially if intended for young children.
- Softness and Texture: Testing the teddy bears for the right level of softness and texture.
- Overall Aesthetic: Assessing the appearance to ensure it meets the desired design criteria.
- Functionality: If the teddy bear includes additional features like sound or movement, these are tested for proper function.
In this example, the QC team plays a crucial role in maintaining the quality of the teddy bears, which directly impacts customer satisfaction and brand reputation. This scenario showcases how meticulous and comprehensive QC processes are essential in delivering a high-quality product.
Quality Control Careers
Quality Control (QC) offers a rewarding career path for those who enjoy working with others, presenting results, and striving for improvements and safety. The qualifications and skills needed for a career in QC vary depending on the industry, but here are some general guidelines:
Educational Requirements
- Entry-level QC positions typically require at least a high school diploma
- A bachelor’s degree may be necessary for more advanced roles, depending on the industry
- Industry-specific background knowledge is often important
Licenses and Certifications
- Some businesses and sectors may require specific licenses and certifications
- Professional development courses and certifications, such as Six Sigma or a Certified Quality Inspector designation, can be beneficial
Skills Needed
- Attention to detail is crucial in QC roles
- Mathematical and mechanical skills are often required
- Physical strength can be important, especially in manufacturing environments
- Technical knowledge relevant to the specific industry is essential
- The ability to perform well under pressure
Career Path
- The career trajectory in QC can vary by industry, but it generally involves gaining years of professional experience in your field
- Initially, you might start as a quality assurance or control associate
- With experience, you could advance to a senior specialist role and lead teams of QC specialists
A career in Quality Control is dynamic and requires a blend of education, specific skills, and continuous learning. It offers opportunities for growth and specialization, particularly for those passionate about ensuring the quality and safety of products and services.
Source: invensislearning.com